News
Take a look at the most recent news articles about patient safety and infection prevention in endoscopy.
18.04.2024
Clinical Services Journal - Advancing endoscopy through collaboration
Collaboration and partnerships are increasing, and the field of endoscopy is no exception. PENTAX Medical and UV Smart have joined forces to address the evolving demands of the sector, including the need for greener endoscopy.
Read more
17.04.2024
Human touch in endoscopy: the everlasting role of reprocessing staff and the importance of training
Ulrike Beilenhoff explains how trainings, industry insights and audits, serve as the cornerstone for maintaining a high-performing reprocessing team and ensuring hygiene standards are consistently met.
Read more
Listen to our new podcast episode
Our two experts reflect on minimizing the risk of infection in endoscope reprocessing, touching on pivotal topics such as the role of manual bedside cleaning, the risk of biofilm formation, how to trace a potential infection and the importance of training.
Episode #8: Minimizing the risk of infection in reprocessing with Ulrike Beilenhoff & Prof. dr. Marco Bruno
Read more
12.01.2024
Event recap – expert takeaway- The importance of manual reprocessing in an era of automatization
Prof. Dr. Marco Bruno discusses the difficulty of endoscope reprocessing and why innovative ways must be sought to continuously improve patient safety. Learn more about marrying manual and automized reprocessing steps.
Read more
11.10.2023
International Hospital & Equipment - Finding the balance: the sustainability journey of PENTAX Medical
How is PENTAX Medical becoming a responsible and accountable corporate citizen? By aligning with the SDGs and implementing new design principles. The journey to a more sustainable future has just begun.
Read more
11.10.2023
International Hospital & Equipment - PENTAX Medical’s AquaTYPHOON™ revolutionises cleaning of endoscopes
Harald Huber speaks with International Hospital about the importance of innovation in healthcare. He describes how the AquaTYPHOONTM is a game-changer with regards to improved efficiency, cost reduction and sustainability for hospitals and their endoscopy suites.
Read more
Listen to our new podcast episode
Leading experts discuss the future of green endoscopy in this exciting new episode. Tune in!
#7: How to become sustainable in endoscopy with Prof. Sebastian, Prof. Pohl and Prof. Bisschops
Read more
10.10.2023
Event recap – expert takeaway- Why should we care about green endoscopy?
Prof. Sebastian discusses the coordinated and sustained efforts that are needed to address climate change, especially in the healthcare sector. Learn why green endoscopy is the future:
Read more
07.09.2023
International Hospital & Equipment- PENTAX Medical presents the AquaTYPHOON™
Paul Caesar and Daniel Vinteler speak about the AquaTYPHOON™, the new automated brushless solution for the pre-cleaning of endoscopes. They explain how this innovation addresses hygiene and sustainability challenges faced by healthcare providers.
Read more
14.06.2023
PENTAX Medical Europe introduces brushless solution for the automated pre-cleaning of endoscopes
PENTAX Medical has launched its new brushless solution for automated pre-cleaning of endoscopes: the AquaTYPHOON™. With this launch, PENTAX Medical takes the lead in standardizing the manual pre-cleaning phase for reusable endoscopes - promoting sustainability and infection prevention in the endoscopy suite.
Read more
07.03.2023
[Italian] Tecnomedicina - PENTAX Medical Europe launches new solution for automatic preliminary cleaning of endoscopes
PENTAX Medical has received CE Mark for its new AquaTYPHOON solution, a medical device used in automatic preliminary cleaning of endoscopes. Read the article from Tecnomedicina in Italian here:
Read more
07.03.2023
PENTAX Medical Europe to launch new solution for the automated pre-cleaning of endoscopes
In association with PlasmaBiotics, PENTAX Medical has gained a CE mark for its new, innovative automated device that provides an alternative to manual pre-cleaning of endoscopes – the AquaTYPHOON™.
Read more
21.02.2023
[Dutch] FMT - Product innovations to find balance between patient safety and the environment
In line with the ESGE and ESGENA position statement, PENTAX Medical is taking steps to make endoscopy more sustainable by reshaping its value chain. Patient safety remains PENTAX Medical's primary objective, against which the sustainability ambitions must be weighed up. Read the article from FMT in Dutch here:
Read more
15.02.2023
Expert sit-down blog series - Endoscopy trends in 2023
Ulrike Beilenhoff, ESGENA Scientific Secretary, shares insights on innovations and opportunities that already shaped and will contribute to further improve patient safety and infection prevention in 2023.
Read more
13.12.2022
Umweltbewusst mit dem PlasmaBAG ECO™
Der neue PlasmaBAG ECO™ für das PlasmaTYPHOON System wird aus 80% recyceltem Polyethylen hergestellt und wird mit dem „Blauen Engel“ ausgezeichnet. Er ist ein Beispiel für die kontinuierliche Entwicklung in Richtung Nachhaltigkeit bei PENTAX Medical.
Read more

14.11.2022
Get practical tips for the processing of flexible endoscopes for free
A successful use and processing of flexible endoscopes are important to minimise damages on endoscopes and maximise patient safety.
Read more
11.11.2022
20 years in Gastrointestinal endoscopy
Dr. Klaus Mergener, global chief medical officer of Pentax Medical, reflects on changes in GI medicine and offers some predictions for the future in the latest publication of the Clinical Services Journal.
Read more
07.11.2022
Expert sit-down blog series - Infection prevention in operating theatres
Dr Mariusz Skrzypczak, MD, Head of the Department of Anaesthesiology and Intensive Care at the Pulmonology and Thoracic Surgery Center in Poznań, Poland, shares his clinical experience with PENTAX Medical ONE Pulmo™ single-use bronchoscope in the ICU.
Read more

14.10.2022
Clinical Services Journal - Advancing endoscopy with Blackbox Innovations
PENTAX Medical’s Harald Huber and Michael Unger discuss key trends and innovation in endoscopy, with The Clinical Services Journal. They highlight some of the key developments that are expected to advance endoscopy in terms of safety, productivity, and clinical outcomes.
Read more

12.10.2022
International Hospital - Empowering physicians with a range of innovative hygiene solutions
In endoscopy, hygiene is both a challenge and a priority. Harald Huber, Global Vice President for Product and Category Management at PENTAX Medical, emphasizes how important it is to provide physicians a meaningful mix of innovative hygiene solutions.
Read more

12.09.2022
Expert sit-down blog series - High quality pulmonary care with single-use scopes
Prof. Felix Herth, Medical Director of the Department of Internal Medicine and Pneumology at the Thoraxklinik Heidelberg, Germany, discusses his clinical experience with PENTAX Medical ONE Pulmo™ single-use bronchoscope and explains how potential vulnerabilities of cross contamination are avoided with this solution.
Read more
01.09.2022
GastroToday - The power of choice: offering the best clinical and environmental solutions for improved patient outcomes
PENTAX Medical is taking ambitious measures to make GI endoscopy more sustainable. Patient safety remains the primary goal of PENTAX Medical, against which sustainability ambitions need to be weighted. So how to balance sustainability goals with patient safety, all the while actually increasing patient outcomes?
Read more

01.05.2022
Clinical Services Journal - Driving innovation in endoscopy
Michael Unger discusses some of the key healthcare challenges driving innovation in the design of endoscopy hygiene solutions and offers an insight into the ways in which industry is striving to help improve sustainability.
Read more

04.04.2022
FDA Recommends Transition to Duodenoscopes with Innovative Designs
The FDA believes the best solution to reducing the risk of disease transmission by duodenoscopes is through innovative device designs that make reprocessing easier, more effective, or unnecessary.
Read more

04.04.2022
Making endoscopy safe and sustainable
European Hospital spoke with two experts at Pentax Medical about challenges in product development, striking the balance between hygiene and sustainability, and the value of training.
Read more

15.03.2022
Interview: International Hospital speaks to PENTAX Medical about their new focus on ‘Power of Choice’
International Hospital’s Twan Heesakkers speaks to Harald Huber, Global VP for Product and Category Management at PENTAX Medical, about the company’s new single-use bronchoscope, what they are focusing on this year and the ‘Power of Choice’.
Read more

01.03.2022
Recap video: PENTAX Medical Hygiene Event 2022
Exclusive footage from the PENTAX Medical Hygiene Event, held on March 1st 2022, on Patient Safety, Hygiene and Sustainability in endoscopy.
Read more

04.02.2022
Patient safety, Hygiene and Sustainability: Discussing solutions for today's challenges in endoscopy
PENTAX Medical is hosting their second online event, during which four key experts in the field will discuss today's challenges in endoscopy. The speakers will reflect on topics such as the Power of Choice in bronchoscopy, the importance of endoscope drying, the necessity and value of training, and sustainable endoscopy. We welcome you to participate in the breakout sessions and continue the discussion. Register now to make your mark on the future of endoscopic hygiene!
Read more

31.01.2022
PENTAX Medical Europe launches premium solution PlasmaTYPHOON+
PENTAX Medical Europe has launched a new ultra-fast endoscope drying and storage solution – the PlasmaTYPHOON™+. Designed to improve productivity and traceability, this premium model further advances endoscope reprocessing to enhance patient safety.
Read more

03.12.2021
PENTAX Medical Europe expands product offering
PENTAX Medical is expanding its offering, to present clinicians and healthcare providers with the power of choice. The portfolio includes the PENTAX Medical ONE Pulmo™ single-use bronchoscope, and will soon include the Slim DEC™ Duodenoscope (ED32-i10; OE-A65), PlasmaTYPHOON™+, and PENTAX Medical DEC™ hygiene pack.
Read more

02.12.2021
Empowering healthcare providers with the power of choice
Providing innovative endoscopic solutions means understanding clinicians’ needs to optimally treat patients, based on their specific condition. PENTAX Medical provides a range of equipment to treat each and every patient in the most optimal way, in order to minimize the risk of infection.
Read more
09.07.2021
PENTAX Medical ONE Pulmo: the single-use bronchoscope enhancing pulmonary care
PENTAX Medical ONE Pulmo is a single-use bronchoscope providing superior suction and HD image quality to help improve pulmonary care. This single-use bronchoscope offers sterility whilst maintaining its high-level HD visualisation, broadening its clinical application beyond that of a standard disposable scope.
Read more

26.05.2021
PENTAX Medical Europe to launch single-use bronchoscope
PENTAX Medical Europe, a leading company in the endoscopic field, has obtained a CE mark for its new single-use bronchoscope – the PENTAX Medical ONE Pulmo. This innovative product provides high quality care without compromise in pulmonary care. The PENTAX Medical ONE Pulmo is a single-use bronchoscope with superior suction power and HD image quality.
Read more

14.04.2021
Improving Hygiene In Endoscopy
At ESGE Days 2021, PENTAX Medical hosted a symposium to hear from key opinion leaders on Scientific Perspectives on Hygiene Innovation in Endoscopy.
Read more
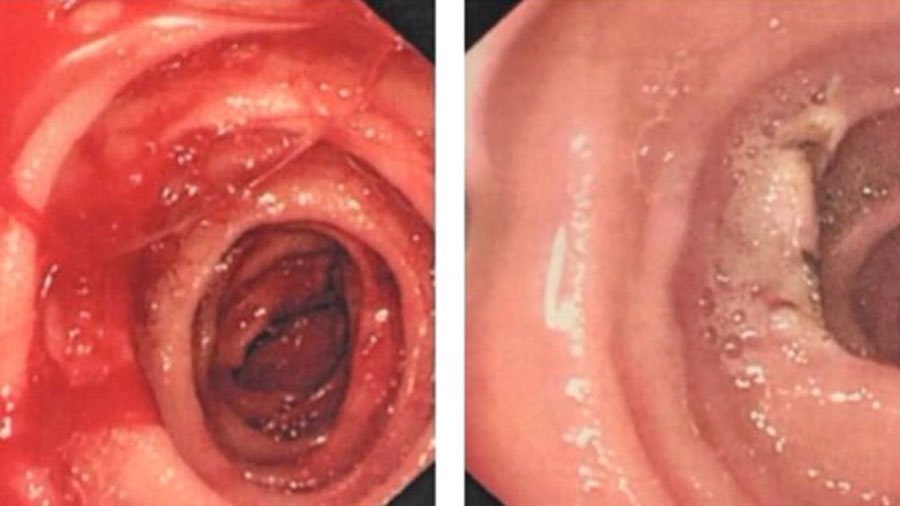
Spring 2021
Capsule captures culprit: Dieulafoy's lesion in the small bowel
A 77 year old female presented to our Emergency Department with a three day history of malaena.
Read more

30.03.2021
Raising patient safety standards during endoscopy
Infection prevention is of paramount importance in the practice of endoscopy.
Read more

30.03.2021
Ways to further improve patient safety in endoscopy
Infection prevention is pivotal in endoscopy. Working closely with experts for market insight is crucial ...
Read more

01.01.2021
Solutions to improve hygiene standards: medical professionals' perspectives
Improving hygiene standards is important to patient safety.
Read more

21.12.2020
Ways to enhance hygiene in endoscopy
Stringent endoscope cleaning between procedures is vital.
Read more

17.12.2020
Solutions to improve hygiene standards
Improving hygiene standards is important to patient safety.
Read more

19.11.2020
Ensuring Patient Safety in Endoscopy
Patient safety in endoscopy must be approached from a holistic perspective ...
Read more
01.09.2020
Ensuring patient safety in endoscopy
Patient safety in endoscopy must be approached from a holistic perspective ...
Read more

01.09.2020
Ensuring Patient Safety In Endoscopy
Patient safety in endoscopy must be approached from a holistic perspective ...
Read more

26.08.2020
Improving hygiene in endoscopy
The use of flexible endoscopes for endoscopic retrograde cholangiopancreatography (ERCP) is increasing ...
Read more
12.06.2020
COVID-19: Ensuring Safety In Endoscopy
How can hospitals ensure they protect patients from the potential risks of infection?
Read more

11.05.2020
COVID-19: PENTAX Medical Talks Supporting Improved Patient Outcomes
How is COVID-19 affecting patient management?
Read more

01.04.2020
A year with covid-19: supporting hospitals with advanced endoscope drying & effective storage
The PlasmaTYPHOON and PlasmaBAG system ...
Read more

19.03.2020
Empowering Reprocessing Staff: Improving Patient Safety
Human factor aspects of reprocessing can never be completely avoided yet staff can be empowered to reduce the risk of infection.
Read more

08.01.2020
Enhancing Patient Safety In Endoscopy
Rainer Burkard, Managing Director of PENTAX Europe, discusses the latest hygiene approaches in endoscopy ...
Read more

28.11.2019
PENTAX Medical DEC™ HD Duodenoscope receives FDA clearance
Conventional duodenoscopes are linked to infection risk, despite proper reprocessing after endoscopic retrograde cholangiopancreatography (ERCP) procedures.
Read more

20.11.2019
QUOTED. 20 November 2019. Jeff Shuren.
The US FDA has cleared the PENTAX Medical Video ED34-i10T2, the first duodenoscope for the US market ...
Read more

19.11.2019
Insights from Expert Round Table @ PENTAX Medical R&D Center Augsburg
Experts in endoscopy: infection risk mitigation in reprocessing deserves more attention and a holistic approach ...
Read more

18.11.2019
FDA Clears First Duodenoscope with Disposable Business End
PENTAX Medical won FDA clearance for its ED34-i10T2 duodenoscope that features a disposable elevator end piece ...
Read more

18.11.2019
Drying in endoscope reprocessing: Essential to patient safety
In practice, the drying of the endoscope is often underestimated and therefore a possible pitfall for hygiene and reprocessing steps.
Read more
18.11.2019
FDA Clears Pentax’s Easier-To-Disinfect Duodenoscope
The US FDA cleared PENTAX Medical’s new duodenoscope as the first in the US with a disposable elevator part that reduces the number of parts needed to be disinfected compared to earlier designs.
Read more

18.11.2019
FDA clears Pentax duodenoscope with disposable part in bid to cut infection risk
FDA cleared the first duodenoscope with a sterile, disposable elevator component Friday in an effort to cut down on the risk of infection from reprocessing the class of devices.
Read more

18.11.2019
FDA authorises Pentax Medical Video Duodenoscope ED34-i10T2
The US Food and Drug Administration (FDA) has given marketing approval for the PENTAX Medical Video Duodenoscope ED34-i10T2 ...
Read more

15.11.2019
FDA clears first duodenoscope with disposable elevator piece, reducing the number of parts needing disinfection
The U.S. Food and Drug Administration today cleared for marketing in the U.S. the first duodenoscope with a sterile, disposable elevator component ...
Read more

15.11.2019
FDA clears Pentax's duodenoscope designed to reduce need for disinfection
The U.S. Food and Drug Administration said on Friday it had cleared medical equipment maker PENTAX of America’s duodenoscope ...
Read more

18.10.2019
Pentax Medical Pushes People, Product And Process Amid New US FDA Guidance
With the US FDA urging providers to transition away from fixed duodenoscopes to newer designs that reduce or eliminate reprocessing of the device ...
Read more

15.09.2019
Infection risk mitigation by simplified IFUs
Duodenoscopes have been long recognized to require precise execution of reprocessing instructions to properly clean and disinfect.
Read more
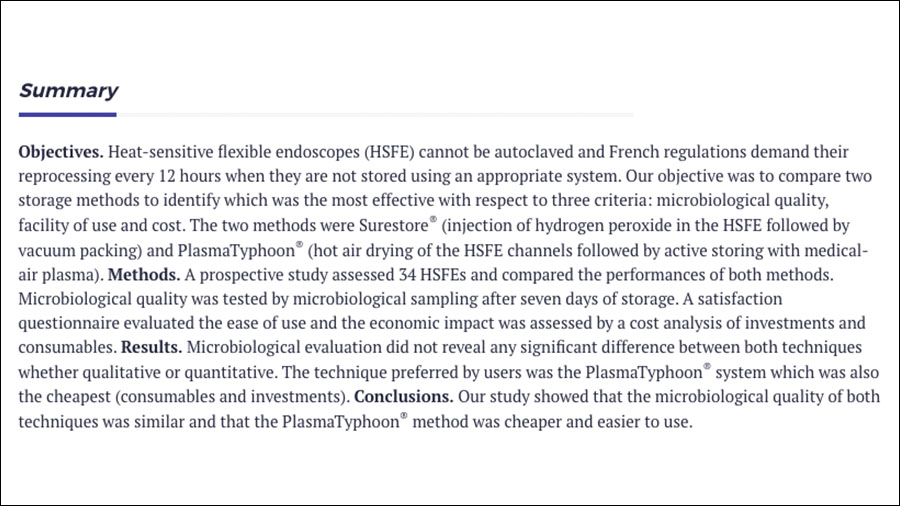
09.09.2019
Comparison of two storage techniques for heat-sensitive flexible endoscopes
Heat-sensitive flexible endoscopes (HSFE) cannot be autoclaved and French regulations demand their reprocessing every 12 hours when they are not stored using an appropriate system.
Read more

01.09.2019
Infection risk mitigation by simplified IFUs
Duodenoscopes have been long recognized to require precise execution of reprocessing instructions to properly clean and disinfect.
Read more

18.07.2019
Limited time and storage space in endoscope reprocessing: A new perspective
In endoscope reprocessing, time and storage space are always limited.
Read more
05.07.2019
The question of immediate reuse of endoscopes after reprocessing
Although the importance of scope reprocessing is widely recognized, there is no clear alignment on certain aspects of the reprocessing steps.
Read more

12.06.2019
Hygiene committed: Pentax Medical addresses the challenges in endoscopy
Recent publications have stated that several outbreaks have been caused by insufficient drying of duodenoscopes.
Read more
01.05.2019
2 Devices That Could Clean Up the Dirty Scope Problem
Boston Scientific is on track to launch a single-use duodenoscope this year designed to eliminate scope disinfection challenges completely.
Read more

24.04.2019
PlasmaTYPHOON Endoscope Drying and Storage Device
PENTAX Medical, a global medical technology developer originally founded in Japan, provides the PlasmaTYPHOON Endoscope Drying and Storage Device through its controlling interest in PlasmaBiotics, a company based in France.
Read more

16.04.2019
PlasmaTYPHOON Makes Sure Endoscopes are Dry and Hygienic
PENTAX Medical, a part of HOYA Group, has unveiled a new endoscope processing and storage solution that helps to eliminate any chance of bacterial infections.
Read more